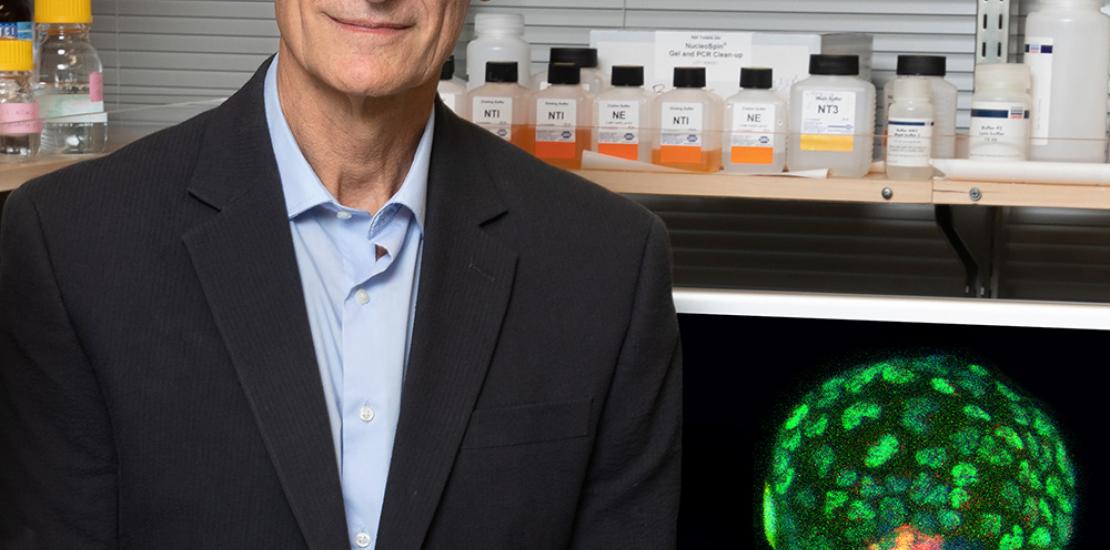
Izpisua creates synthetic mouse embryos to gain more knowledge about the beginning of life and human health
They are mouse embryods (in the blastocyst phase) for whose creation he has used iPS cells. The advance will allow to study, without having to use natural embryos nor gametes, the development of the first embryonic stages, the causes of miscarriages or the onset of diseases such as Alzheimer’s disease, and it will provide valuable information for the creation of human organs for transplants. The research has been developed by the team of scientists led by Dr. Juan Carlos Izpisua, director of the Gene Expression Laboratory of the Salk Institute, in the US, and Professor of Developmental Biology at the UCAM.
Some of the major events in the life of human beings do not only take place before birth, but during the first days, after the fertilisation of the egg by the sperm cell. The way the first 100 cells organise themselves after the division of the zygote to form the blastocyst has deep implications for whether a pregnancy is successful, how organs form, and even for the onset of diseases, such as Alzheimer’s disease, in later stages of life. Nevertheless, up until now, scientist have not been able to study in detail how a blastocyst is formed because they lacked the adequate models to do so.
For the first time, a team of researchers, led by Dr. Izpisua Belmonte, has created blastocyst-like structures from mouse, called “blastoids”, starting from the in vitro culture of one cell, thus avoiding the use of natural embryos to carry out this type of studies. As reported by the journal Cell, these “blastoids” have the same structure as natural blastocysts and they could be used not only to gain more knowledge about early embryonic development, but also to study the problems associated with pregnancy, infertility or illnesses that may appear at different stages of life. “These studies will help us to better understand the very beginnings of life; how early on in life a single cell can give rise to millions of cells and how they are assembled in space and time to give rise to a fully developed organism. Importantly, this work avoids the use of natural embryos”, states Dr. Izpisua Belmonte, Professor of the Gene Expression Laboratory of the Salk Institute, in the US, and Professor of Developmental Biology at the UCAM.
Very little is known on the development of natural blastocysts, since the animal models, such as mice, produce a small quantity of these structures and, therefore, it is not easy to test the effects, on their development, of malnutrition, exposure to toxins or of a variety of genetic and epigenetic mutations. “This work will enable important research into early developmental defects”, says professor Jun Wu, one of the authors of the study.
The team of researchers developed the “blastoids” by using mouse embryonic cells and, more importantly, adult mouse cells. First of all, to do so, the adult cells were converted into induced pluripotent stem cells or iPS.
To induce the iPS cells to form “blastoids”, they were put into a chemical solution to increase their ability to develop and, subsequently, they were cultivated in small groups in a special culture medium where they soon formed connections with each other and then, the connected cells started to create a sphere with an internal and an external layer. The cells facing inward accumulated proteins that made them distinct from the cells facing outwards, which started to activate a key protein for the assembly of the natural blastocyst, called YAP, which entered the cell nucleus and induced the expression of proteins to build what might eventually become a placenta. “The formation of “blastoids” mimics the natural developmental process”, points out Ronghui Li, co-author of the study.
The “blastoids” contained the three types of primordial cells (from which all the cells of an adult organism derive, including the placenta and its products) that can be found in natural blastocysts. They also had a size and a gene expression profiling similar to the one of natural blastocysts. “I think that this model will be very powerful to study the early embryonic development in mammals”, says Cuiqing Zhong, co-author of the article.
Now, the research team is working to combine the cutting-edge gene-editing tools, such as CRISPR-Cas9, with their new “blastoid” model, to study how genetic changes, which lead to miscarriage, affect the composition and the development of the three types of different cells of the “blastoid”. The “blastoids” are also useful as a model to test drugs and chemical products for future therapies. “The creation of these “blastoids”, which does not only avoid the use of natural embryos but also the use of gametes, will allow us to study the most important stages of the embryonic development of an organism and, therefore, we are convinced that it will have deep implications for improving human health”, says Estrella Núñez, co-author of the article and vice-chancellor for Research at the UCAM, the entity that promotes the research.
The generated “blastoids” cannot convert into functional embryos; their cells grow into disorganised tissue. The researchers believe that, by working on the optimisation of the process, they will be able to obtain completely functional “blastoids”, able to develop until the stages where the different organ primordia are formed and thus to be the seed of “organoids” that could be used as a source for organ transplantation. “With enough time and resources, these first studies will have far-reaching implications for human health”, says Wu.
This work has been financed by the National Key R&D Program of China (2016YFC1000601), the G. Harold and Leila Y. Mathers Charitable Foundation, The Moxie Foundation, The Leona M. and Harry B. Helmsley Charitable Trust (2012-PG-MED002), The Hewitt Foundation, the National Institutes of Health (5 DP1 DK113616) and the Catholic University of Murcia (UCAM)